.webp?width=900&name=thumbnail_large-1(5).webp)
PGx Testing Becoming a New Standard - Studies Show a Savings of Lives
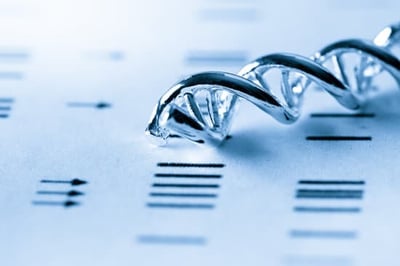
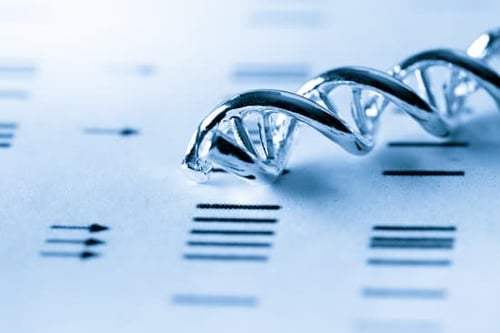
Pharmacogenomics (PGx) testing is becoming a new standard of care when prescribing medications about how inherited genetic differences affect individual responses to drugs, both in terms of therapeutic effects as well as adverse effects.
The idea of pharmacogenomics, often used interchangeably with the term pharmacogenetics, is not, however, a new idea with Greek philosopher Pythagoras, an influencer of Plato, Aristotle, and western ideals, touching upon it in 510 B.C.
“The history of pharmacogenetics stretches as far back as 510 B.C. when Pythagoras noted that ingestion of fava beans resulted in a potentially fatal reaction in some, but not all, individuals,” wrote Sir Munir Pirmohamed, a clinical pharmacologist and geneticist, in the British Journal of Clinical Pharmacology.
The promise that pharmacogenomics can help prevent adverse drug events (ADEs) while making medication administration more efficient by focusing on drug metabolization is becoming a reality as recent studies are showing that PGx can save both lives and costs.
And the future holds untapped potential as PGx testing could unlock the world of molecular diagnostics for the general population.
Pharmacogenomics Can Prevent Adverse Drug Events
Pharmacogenomics is the study of the role of the genome in drug response and analyzes how the genetic makeup of an individual affects their response to drugs.
“Pharmacogenomics (PGx) is the study and clinical application of the role of genetic variation on drug response. Mounting evidence suggests PGx can improve the safety and/or efficacy of several medications commonly prescribed in primary care,” said a study published in Genes (Basel).
The Journal of Personalized Medicine published an article that said PGx has been shown to play an important role in certain prescribing situations by identifying medication responders and non-responders to avoid specific adverse events and optimize drug doses.
“When we are sick, we seek relief as quickly as possible. Fortunately, medical science has developed extensive pharmacopeia for treating a very large number of pathologies. Although popping a pill, drinking an elixir, or getting an injection often provides relief or a cure, these solutions can come with side effects. One of the side effects that have the highest impact on the patient and healthcare costs is when a drug produces an unwanted effect or injury – also known as an adverse drug event (ADE),” says the Behind the Bench publication.
Adverse drug effects, annually, according to the US Health and Human Services (HHS) Office of Disease Prevention and Health Promotion:
- Account for an estimated 1 in 3 of all hospital adverse events.
- Affect about 2 million hospital stays each year.
- Extend hospital stays by 1.7 to 4.6 days.
- Account for over 3.5 million physician office visits.
- Result in an estimated 1 million emergency department visits.
- Drive approximately 125,000 hospital admissions.
The Link Between Drug Metabolism and Genotypes
PGx also plays a significant role in exploring the link between drug metabolism and genotypes.
“A less obvious problem is that the response to medicine often depends on the genotype of the recipient. Most modern drugs are acted on by drug metabolizing enzymes (DMEs),” says the Behind the Bench article. “Enzymes that fall into this group are required to clear the drug from the system or are required to convert a precursor molecule into a biologically active molecule. However, not all individuals are equipped with the same set of DMEs.”
The outcomes, according to the Behind the Bench staff, that can happen included:
- Individuals that completely lack functional copies of the enzymes needed to convert a drug precursor into the biologically active molecule, rendering the medication ineffective.
- Individuals with enzymes with lower functional activity, meaning the precursor molecule is converted to the functional drug at a lower rate, effectively lowering the treatment dose.
- Individuals with a lower activity of the enzymes that degrade a drug, resulting in higher circulating concentrations of the biologically active molecule.
- Individuals that may convert a drug into a toxin product because of their inborn metabolism.
- Individuals that might be more sensitive to drug side effects because of their inborn metabolism.
“Pharmacogenomics is the field that analyzes the link between DME genotypes and drug metabolism. There is increasing interest among patients, medical practitioners, governmental health services, and insurance providers to use pharmacogenomics to increase the effectiveness and safety of drug treatments. Finding the most effective, safe, and economical treatment options involves making sure the recipient’s genotype can metabolize the drug as intended,” concluded the Behind the Bench staff.
PGx Can Advance the Field of Molecular Diagnostics
John Howe, Ph.D., director of the molecular diagnostics laboratory at Yale School of Medicine, explains that PGx could fuel the expansion of molecular diagnostics.
“Molecular diagnostics, also called molecular pathology, involves taking DNA or RNA, the unique genetic code found in our cells and analyzing the sequences for red flags that can pinpoint the potential emergence of a specific disease. The field has expanded rapidly in recent years,” writes Howe.
Howe says that when he joined the field in the 1990s, there were just a few molecular diagnostic tests, but now there are tests in most of the areas of pathology.
Tests are typically performed to determine whether patients have a gene mutation associated with a specific disease, either as an inherited or an acquired mutation. Inherited diseases can be tested for at the prenatal, newborn, and adult stages of life.
Howe described specific situations where PGx is being employed:
- Doctors performing molecular tests for a common inherited hereditary cancer. For example, in breast cancer, they can investigate for specific inherited mutations in the BRCA1 and BRCA2 genes, which may increase the patient's risk of breast and ovarian cancer.
- Acquired gene mutations can be tested for in some cases, such as for chronic myeloid leukemia (CML). A patient can then start therapy as soon as possible.
- Tests can also be done to determine whether a person has become resistant to a specific drug and needs to change course in a treatment regimen. For example, an HIV patient can be monitored by a quantitative molecular test to determine whether or not the amount of viral load has significantly increased, which is a sign of resistance to the treatment. The patient’s HIV can then be DNA sequenced to determine if a mutation known to be associated with resistance is found.
Pharmacogenetics: Saving Lives and Costs
While many have conceded to the benefits of pharmacogenomics and pharmacogenetics, there was some hesitation that the costs associated with the testing justified the results. However, recent studies are making the case that PGx saves not only costs but also lives.
A research paper published in Frontiers in Pharmacology in June 2022 showed the cost-effectiveness of pharmacogenomics-guided prescribing to prevent gene-drug-related deaths.
“We used a decision-analytic model to quantify the number and cost per gene-drug-related death prevented, from a 1-year Dutch healthcare perspective. The modeled intervention is a single gene PGx-test for CYP2C19, DPYD, TPMT, or UGT1A1 to guide prescribing based on the DPWG recommendations among patients in the Netherlands initiating interacting drugs (clopidogrel, capecitabine, systemic fluorouracil, azathioprine, mercaptopurine, thioguanine or irinotecan),” wrote the paper’s authors.
The results were stunning:
- For the 148,128 patients initiating one of the seven drugs in a given year, costs for PGx-testing, interpretation, and drugs would increase by 21.4 million euros.
- 35,762 (24.1 percent) of these drug initiators would require an alternative dose or drug.
- PGx-guided prescribing would relatively reduce gene-drug mortality by 10.6 percent and prevent 419 deaths a year (0.3 percent of initiators).
- Cost-effectiveness is estimated at 51,000 euros per prevented gene-drug-related death.
Another recent study, in Neuropsychiatric Disease and Treatment, found that pharmacogenetic testing for depression in real-world clinical settings saved each patient $3,962 per year, after considering the cost of genetic testing.
“Our study utilizes published health care costs and clinical patient outcome data to model the economic impact of pharmacogenetic-guided treatment for depression in a variety of clinical settings. Assuming a test cost of $2,000 for pharmacogenetic testing, the model predicts a savings of $3,962 annually per patient with pharmacogenetic-guided medication management,” said the study.
Studies like these make it more likely that healthcare providers, insurers, and patients will opt for more PGx in the future.
“A much-cited barrier for the implementation of personalized medicine is the lack of cost-effectiveness studies to assess the economic benefit of pharmacogenetic-guided medication management in a “real-world” environment. Our model presents significant cost savings of pharmacogenetic testing in a diverse set of clinical settings and health care providers,” said the study authors.
Another study, in the Journal of Personalized Medicine, published in March 2022, says that PGx benefits could help healthcare across multiple levels.
“Indeed, outcome-oriented studies repeatedly demonstrate positive impacts of PGx on individuals, providers, and the healthcare system. Oncology and psychiatry implementations demonstrate particularly well-documented improvements. Further, a variety of positive economic, clinical, and humanistic outcomes (ECHO) associated with PGx have also been described. Thus, while implementational challenges exist, it is clear that PGx enhances healthcare delivery at multiple levels,” said the authors.
Subscribe to email updates
Related Articles
Topics
Topics

Topics